If you’re in Year 10 and the word “moles” makes you think of tiny furry animals rather than chemistry equations... you’re not alone! Quantitative Chemistry can feel like a mountain of numbers, but with the right approach (and maybe some expert tuition 😉), it can start to make sense – even feel fun!
At Capital Tuition Group, we’ve helped hundreds of students across the UK tackle tough GCSE topics like Quantitative Chemistry with confidence. So, let’s break it down.
At Capital Tuition Group, we’ve helped hundreds of students across the UK tackle tough GCSE topics like Quantitative Chemistry with confidence. So, let’s break it down.
🧪 What is Quantitative Chemistry?
Quantitative Chemistry is all about the numbers behind chemical reactions. Think of it as the maths that tells us how much of each substance is involved in a reaction.
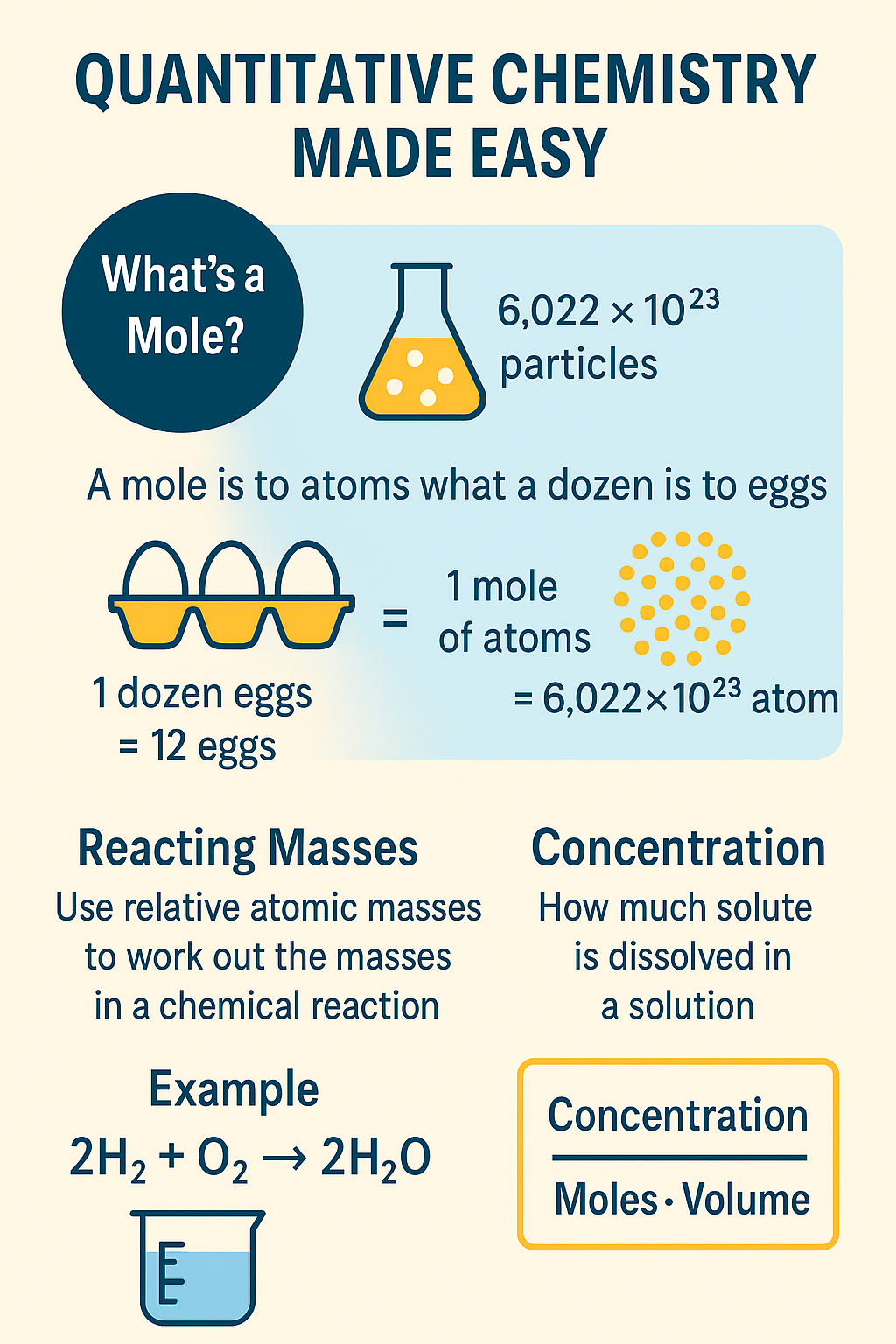
The key ideas include:
- Moles (not the animal kind!)
- Reacting masses
- Concentration calculations
🐾 1. What’s a Mole? (No, Not That Kind)
In Chemistry, a mole is a huge number – 6.022 × 10²³ to be exact (also known as Avogadro’s Number). That’s how many particles (atoms, molecules or ions) are in one mole of a substance.
Think of it like this:
A mole is to atoms what a dozen is to eggs.
🥚 1 dozen eggs = 12 eggs
🧪 1 mole of atoms = 6.022 × 10²³ atoms
So when you hear “moles,” think counting particles.
⚖️ 2. Reacting Masses – It’s Like a Chemical Recipe
Imagine you’re baking a cake. The recipe says you need:
- 200g flour
- 100g sugar
- 2 eggs
In chemistry, equations are like recipes too! They tell us how much of each chemical we need and what we’ll get out.
Example:
If the reaction is:
2H₂ + O₂ → 2H₂O
This tells us:
- 2 moles of hydrogen react with 1 mole of oxygen
- To make 2 moles of water
We can use the relative atomic masses (RAMs) to work out how much mass is involved. It’s like weighing out the right ingredients for a perfect reaction!
💧 3. Concentration – How Strong is Your Solution?
Concentration is all about how much solute (stuff) is dissolved in a certain volume of solvent (usually water). It’s measured in:
mol/dm³ → moles per cubic decimetre (1 dm³ = 1000 cm³)
Formula you need to know:
Concentration = Moles ÷ Volume
If you’ve ever made squash too weak or too strong, you’ve already experienced concentration problems!
🧠 Top Tips to Master Quantitative Chemistry
- Learn your formulas off by heart – flashcards help!
- Practice rearranging equations – it’s a skill, not magic
- Don’t fear units – always check you're using the right ones (e.g., cm³ vs dm³)
- Use real-life analogies – cooking, baking, and even Lego can help make things click
- Ask for help when it doesn’t make sense – you’re not alone!
📚 Still Confused? Let Capital Tuition Group Help!
If your child finds chemistry more confusing than clear, it’s not a failure – it’s a sign they need a new approach.
At Capital Tuition Group, we match students with qualified, experienced science tutors who break down tricky topics like Quantitative Chemistry into bite-sized, understandable chunks. Whether your child needs exam prep, topic-specific support, or a long-term confidence boost, we’ve got you covered.
✅ One-to-one or small group lessons
✅ Online or in-person across the UK
✅ All tutors are DBS-checked Qualified Teachers and subject specialists
💬 Let’s Get Started
Want your child to feel more confident in Chemistry?
Get in touch with Capital Tuition Group today – we’ll find the perfect tutor to help them master the mole and conquer GCSE Science with confidence.
👉 For One-To-One Tuition contact us here.
👉 For Group Tuition, sign up to our Weekly Term-Time Year 10 Tuition Classes, or our May Half Term Year 10 GCSE Science Revision Boosters
👉 For Group Tuition, sign up to our Weekly Term-Time Year 10 Tuition Classes, or our May Half Term Year 10 GCSE Science Revision Boosters